
If the layers increase, the atomic size will also increase. The size of an atom depends on the electrons present in the outer layers. Due to the increased nuclear charge, there will be an electron attraction with greater force. If the value of the nuclear charge is higher, then the value of electronegativity is also greater. The higher the number of protons inside the nucleus, the higher the nuclear charge. The following are some of them:Ī nuclear charge simply means the charge of protons present in the nucleus of an atom. Various phenomena influence electronegativity. Electronegativity can be measured on different scales however, the most common scale used to measure is the one designed by Linus Pauling. The main purpose of electronegativity is to indicate the net outcomes of atoms’ tendencies in multiple elements to attract the electron pairs that possess bond-forming. Electronegativity is a dimensionless property because it does not involve any dimension and is only a capability. It is nothing but the tendency of an atom to attract the electron shared pairs towards itself is called electronegativity. Furthermore, ionic compounds are compounds formed by electrostatic attraction of negative and positive ions.On the other hand, when the difference is smaller, covalent bonds are formed. When the atoms consist of huge differences in electronegativity, ionic bonds are formed.This force is called an electrovalent or ionic bond.

Usually, electrovalent bonds are formed only between non-metals and metals. They will contain positive and negative ions. These bonds are referred to as electrovalent bonds or ionic bonds.
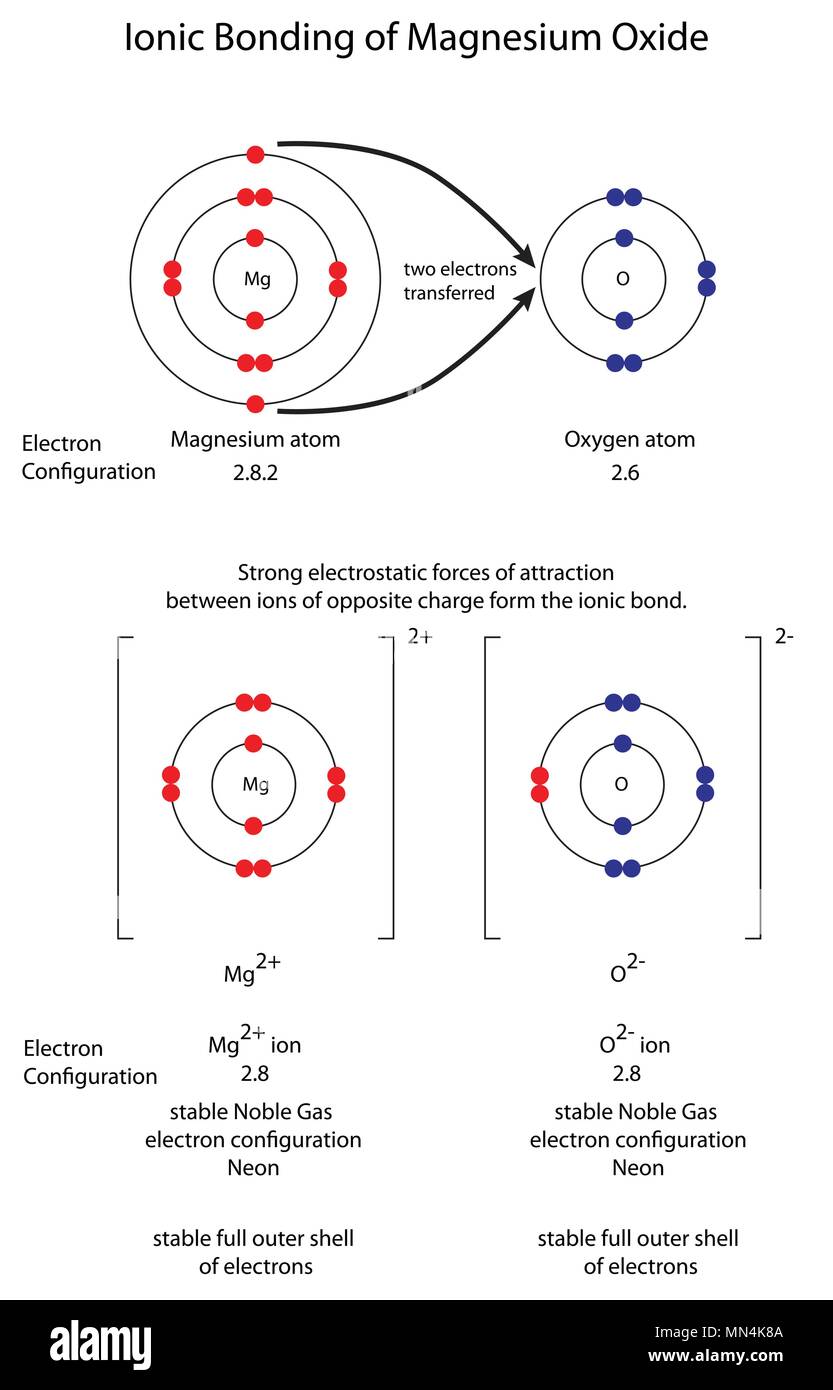
While transferring electrons from one atom to another, bonds are formed. Let us have a look at the ionic bonding diagram below:įrom this diagram, one can easily understand that an ionic molecule is formed when a metal atom transfers its electrons to a non-metal atom. It happens in the outermost layers of an atom. In the majority of cases, one atom loses electrons while the other gains them. Hence, a bond formed due to this type of configuration is called the electrovalent or ionic bond. The most commonly used way is when atoms donate or accept electrons from neighbouring atoms to accomplish their octet configuration. In order to lose energy to become stable, there are three ways the atoms could follow. Due to this bondage, the atoms are capable of obtaining their inert gas configuration. Simply put, a chemical bond will be formed among two atoms by transferring one or more electrons from one atom to another. When two or more oppositely charged ions are held together due to the presence of electrostatic force, the resulting bond is termed an ionic bond. This article will provide further knowledge regarding ionic bonds while having a good look at other related concepts. So, this is what happens inside an ionic bond. After that, using that atom bondage as a bridge, electrons are transferred from one atom to another. It starts by forming a bond between one or more than one atoms. In ionic bonding, a simple process takes place. It is a fundamental topic that every chemistry enthusiast should know. What is an ionic bond? How do we hold different ions together? We may have come across such questions while dealing with the Chemistry subject.


 0 kommentar(er)
0 kommentar(er)
